3个回答
法规和指南中明确要求需要培养后观察,培养期间的观察没有明确的规定,企业可基于风险自行制定,另外培养前需要移除密封性有问题缺陷瓶,培养前后要保持物料平衡。
EU GMP ANNEX 1 培养后观察
9.45 On completion of incubation:
- i. Filled APS units should be inspected by personnel who have been appropriately trained and qualified for the detection of microbiological contamination. Inspection should be conducted under conditions that facilitate the identification of any microbial contamination.
- ii. Samples of the filled units should undergo positive control by inoculation with a suitable range of reference organisms and suitably representative local isolates.
-
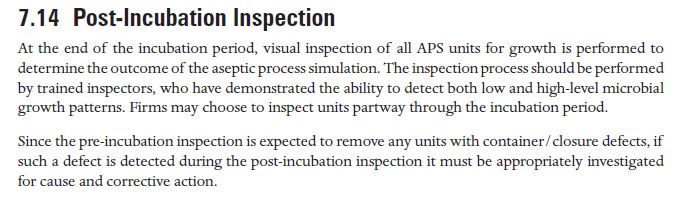
- PDA TR 22 培养后观察
-
-
评论
匿名
提交
取消
匿名
{{item_parent.created_at}}
置顶
批准
驳回
编辑
等待审核
已驳回
回复
{{item_parent.show_reply_list ? '收起回复' : '查看回复'}}({{item_parent.children.length}})
编辑
提交
取消
写回复
匿名
提交
取消
{{item_children.from_user}} 回复 {{item_children.to_user}}
{{item_children.created_at}}
批准
驳回
编辑
等待审核
已驳回
回复
编辑
提交
取消
写回复
匿名
提交
取消
这{{threadTextType}}正{{isAdminText}}
举报
提交
取消
为帮助审核人员更快处理,请填写举报原因:
举报
提交
取消
为帮助审核人员更快处理,请填写举报原因:


