3个回答
前段时间刚整理的,Q1比较早了,时间也没找到,其他的如下表,可惜评论区无法添加文件,只能表格形式贴在这里了
| 序号 | 英文题目 | 中文译文 | 阶段 | 发布时间 | 是否有中文译稿 | 中国发布时间 | 中国执行时间 |
| 质量(Quality Guidelines) | |||||||
| 1 | Q1 Stability/稳定性 | ||||||
| Q1A(R2): Stability Testing of New Drug Substances and Products | Q1A(R2):新原料药和制剂的稳定性试验 | 阶段5 | 2003.2.6 | 有 | |||
| Q1B: Stability Testing: Photostability Testing of New Drug Substances and Products | Q1B: 稳定性试验:新原料药和制剂的光稳定性试验 | 阶段5 | 1996.11.6 | 有 | |||
| Q1C: Stability Testing for New Dosage Forms | Q1C:新剂型的稳定性试验 | 阶段5 | 1996.11.6 | 有 | |||
| Q1D: Bracketing and Matrixing Designs for Stability Testing of New Drug Substances and Products | Q1D:新原料药和制剂稳定性试验的括号法和矩阵法设计 | 阶段5 | 2002.2.7 | 有 | |||
| Q1E: Evaluation for Stability Data | Q1E:稳定性数据的评价 | 阶段5 | 2003.2.6 | 有 | |||
| 2 | Q2 Analytical Validation/分析方法验证 | ||||||
| Q2(R1): Validation of Analytical Procedures Text and Methodology | Q2(R1):分析方法论证:正文和方法学 | 阶段5 | 2005.11 | 有 | 2020-01-10 | 2020-07-10 | |
| 3 | Q3A - Q3D Impurities/杂质 | ||||||
| Q3A(R2): Impurities in New Drug Substances | Q3A(R2):新原料药中的杂质 | 阶段5 | 2006.10.25 | 有 | 2020-01-10 | 2020-07-10 | |
| Q3B(R2): Impurities in New Drug Products | Q3B(R2):新药制剂中的杂质 | 阶段5 | 2006.6.2 | 有 | 2020-01-10 | 2020-07-10 | |
| Q3C(R8) Impurities:Guideline for Residual Solvents | Q3C(R8):杂质:残留溶剂的指导原则 | 阶段5 | 2021.4.22 | 有 | 2021-12-20 | 2022-02-20 | |
| Q3D(R2): Guideline for Elemental Impurities | Q3D(R2):元素杂质指导原则 | 阶段5 | 2022.4.26 | 有 | 2020-01-10 | 2020-07-10 | |
| 4 | Q4 - Q4B Pharmacopoeias/药典 | ||||||
| Q4B: Evaluation and Recommendation of Pharmacopoeial Texts for Use in the ICH Regions | Q4B:ICH区域所用药典文本的评价和建议 | 阶段5 | 2007.11.1 | 有 | |||
| Q4B Frequently Asked Questions | Q4B:常见问题与解答 | 2012.4.26 | |||||
| Q4B Annex 1 (R1): Residue on Ignition/Sulphated Ash General Chapter | Q4B附录1(R1): 关于灼烧残渣/灰分 常规篇 | 阶段5 | 2010.9.27 | 有 | |||
| Q4B Annex 2 (R1): Test for Extractable Volume of Parenteral Preparations General Chapter | Q4B附录2(R1): 关于注射剂可提取容量测试 常规篇 | 阶段5 | 2010.9.27 | 有 | |||
| Q4B Annex 3 (R1): Test for Particulate Contamination: Sub-Visible Particles General Chapter | Q4B附录3(R1): 关于颗粒污染物测试:不溶性微粒 常规篇 | 阶段5 | 2010.9.27 | 有 | |||
| Q4B Annex 4A (R1): Microbiological Examination of Non-Sterile Products: Microbial Enumeration Tests General Chapter | Q4B附录4A(R1):非无菌药品的微生物检查:微生物计数试验 常规篇 | 阶段5 | 2010.9.27 | 有 | |||
| Q4B Annex 4B (R1): Microbiological Examination of Non-Sterile Products Tests for Specified Micro-Organisms General Chapter | Q4B附录4B(R1): 非无菌产品的微生物检查—特定微生物 常规篇 | 阶段5 | 2010.9.27 | 有 | |||
| Q4B Annex 4C (R1): Microbiological Examination of Non-Sterile Products: Acceptance Criteria for Pharmaceutical Preparations and Substances for Pharmaceutical Use General Chapter | Q4B附录4C(R1): 非无菌产品的微生物检查:药物制备以及药物使用物质的接受标准 常规篇 | 阶段5 | 2010.9.27 | 有 | |||
| Q4B Annex 5 (R1): Disintegration Test General Chapter | Q4B附录5(R1):崩解试验 常规篇 | 阶段5 | 2010.9.27 | 有 | |||
| Q4B Annex 6 Uniformity of Dosage Units General Chapter | Q4B附录6: 统一剂量单位 常规篇 | 阶段5 | 2013.11.13 | ||||
| Q4B Annex 7 (R2): Dissolution Test General Chapter | Q4B附录7(R2): 溶出试验 常规篇 | 阶段5 | 2010.11.11 | 有 | |||
| Q4B Annex 8 (R1): Sterility Test General Chapter | Q4B附录8(R1): 无菌试验 常规篇 | 阶段5 | 2010.9.27 | 有 | |||
| Q4B Annex 9 (R1): Tablet Friability General Chapter | Q4B附录9(R1): 片剂易碎性 常规篇 | 阶段5 | 2010.9.27 | 有 | |||
| Q4B Annex 10 (R1): Polyacrylamide Gel Electrophoresis General Chapter | Q4B附录10(R1): 聚丙烯酰胺凝胶电泳 常规篇 | 阶段5 | 2010.9.27 | 有 | |||
| Q4B Annex 11: Capillary Electrophoresis General Chapter | Q4B附录11:毛细管电泳 常规篇 | 阶段5 | 2010.6.9 | 有 | |||
| Q4B Annex 12: Analytical Sieving General Chapter | Q4B附录12:分析筛选 常规篇 | 阶段5 | 2010.6.9 | 有 | |||
| Q4B Annex 13: Bulk Density and Tapped Density of Powders General Chapter | Q4B附录13:粉末的堆密度和振实密度 | 阶段5 | 2012.6.7 | 有 | |||
| Q4B Annex 14: Bacterial Endotoxins Test General Chapter | Q4B附录14:细菌内毒素试验 常规篇 | 阶段5 | 2012.10.18 | 有 | |||
| 5 | Q5A - Q5E Quality of Biotechnological Products/生物技术产品质量 | ||||||
| Q5A(R1): Viral Safety Evaluation of Biotechnology Products Derived from Cell Lines of Human or Animal Origin | Q5A(R1):来源于人或动物细胞系的生物技术产品的病毒安全性评价 | 阶段5 | 1999.9.23 | 有 | 2020-01-10 | 2020-07-10 | |
| Q5B: Analysis of the Expression Construct in Cells Used for Production of r-DNA Derived Protein Products | Q5B:源自重组DNA技术的蛋白质产品的表达载体分析 | 阶段5 | 1995.11.30 | 有 | 2020-01-10 | 2020-07-10 | |
| Q5C: Stability Testing of Biotechnological/Biological Products | Q5C:生物技术生物制品质量:生物技术/生物制品稳定性试验 | 阶段5 | 1995.11.30 | 有 | 2020-01-10 | 2020-07-10 | |
| Q5D: Derivation and Characterisation of Cell Substrates Used for Production of BiotechnologicalBiological Products | Q5D: 用于生产生物技术/生物产品的细胞底物的起源和特征描述 | 阶段5 | 1997.7.16 | 有 | 2021-04-28 | 2021-10-28 | |
| Q5E: Comparability of BiotechnologicalBiological Products Subject to Changes in their Manufacturing Process | Q5E:生物技术产品/生物制品在生产工艺变更前后的可比性 | 阶段5 | 2004.11.18 | 有 | 2020-01-10 | 2020-07-10 | |
| 6 | Q6A- Q6B Specifications/规格 | ||||||
| Q6A: Specifications: Test Procedures and Acceptance Criteria for New Drug Substances and New Drug Products: Chemical Substances | Q6A:质量标准:新原料药和新药制剂的检测方法和可接受标准:化学药物 | 阶段5 | 1999.10.6 | 有 | 2020-01-10 | 2020-07-10 | |
| Q6B: Specifications: Test Procedures and Acceptance Criteria for Biotechnological/Biological Products | Q6B: 质量规格:生物技术/生物产品的检验程序和可接收标准 | 阶段5 | 1999.3.10 | 有 | |||
| 7 | Q7 Good Manufacturing Practice/GMP | ||||||
| Q7: Good Manufacturing Practice Guide for Active Pharmaceutical Ingredients | Q7: 原料药GMP指南 | 阶段5 | 2000.11.10 | 有 | 2020-01-10 | 2020-07-10 | |
| Q7 Questions and Answers | Q7 问答部分 | 阶段5 | 2015.6.10 | ||||
| 8 | Q8 Pharmaceutical Development/药物研发 | ||||||
| Q8(R2): Pharmaceutical Development | Q8(R2):药品研发 | 阶段5 | 2009.8 | 有 | 2020-01-10 | 2020-01-10 | |
| Q8, Q9 and Q10 Questions & Answers (R4) | 关于Q8、Q9和Q10的问与答(R4) | 阶段5 | 2010.11.11 | 有 | 2020-01-10 | 2020-01-10 | |
| 9 | Q9 Quality Risk Management/质量风险管理 | ||||||
| Q9(R1):Quality Risk Management | Q9(R1):质量风险管理 | 阶段5 | 2023.1.18 | 有 | 2023-09-04 | 2024-03-04 | |
| 10 | Q10 Pharmaceutical Quality System/药物质量体系 | ||||||
| Q10: Pharmaceutical Quality System | Q10:药品质量体系 | 阶段5 | 2008.6.4 | 有 | 2020-01-10 | 2020-01-10 | |
| 11 | Q11 Development and Manufacture of Drug Substances/化学药品的研发与生产 | ||||||
| Q11: Development and Manufacture of Drug Substances (Chemical Entities and Biotechnological/Biological Entities) | Q11:原料药开发和生产(化学实体和生物技术/生物实体药物) | 阶段5 | 2012.5.1 | 有 | 2020-01-10 | 2020-01-10 | |
| Q11:Questions and Answers | Q11问答:原料药开发和生产(化学实体和生物技术/生物实体药物)问答 | 阶段5 | 2017.8.23 | 有 | 2020-01-10 | 2020-01-10 | |
| 12 | Q12 Techinical And Regulatory Considerations For Pharmaceutical Product Lifecycle Management药品生命周期管理的技术和监管考虑 | ||||||
| Q12:Techinical And Regulatory Considerations For Pharmaceutical Product Lifecycle Management | Q12:药品生命周期管理的技术和监管考虑 | 阶段5 | 2019.11.20 | 有 | 2023-08-25 | 2025-08-25 | |
| Q12 Annexes | Q12附件 | 阶段5 | 2019.11.20 | 有 | |||
| 13 | Q13:Continuous Manufacturing of Drug Substances and Drug Products原料药和制剂的连续制造 | ||||||
| Q13:Continuous Manufacturing of Drug Substances and Drug Products | Q13:原料药和制剂的连续制造 | 阶段5 | 2022.11.16 | 有 | 2023-12-12 | 2024-06-13 | |
| 安全性(Safety Guidelines) | |||||||
| 1 | S1A - S1C Carcinogenicity Studies/致癌性研究 | ||||||
| S1A: Need for Carcinogenicity Studies of Pharmaceuticals | S1A:药物致癌性试验必要性指导原则 | 阶段5 | 1995.11.29 | 有 | 2019-11-05 | 2020-05-01 | |
| S1B: Testing for Carcinogenicity of Pharmaceuticals | S1B:药物致癌性试验 | 阶段5 | 1997.7.16 | 有 | 2019-11-05 | 2020-05-01 | |
| S1B(R1):TESTING FOR CARCINOGENICITY OF PHARMACEUTICALS | S1B(R1):药物致癌性试验 | 阶段4 | 2022.8.4 | 有 | 2023-03-20 | 2023-03-22 | |
| S1C(R2): Dose Selection for Carcinogenicity Studies of Pharmaceuticals | S1C(R2):药物致癌性试验的剂量选择 | 阶段5 | 2008.3.11 | 有 | 2019-11-05 | 2020-05-01 | |
| 2 | S2 Genotoxicity Studies/基因毒性研究 | ||||||
| S2(R1): Guidance on Genotoxicity Testing and Data Interpretation for Pharmaceuticals Intended for Human Use | S2(R1):人用药物遗传毒性试验和结果分析指导原则 | 阶段5 | 2011.11.9 | 有 | 2019-11-05 | 2020-05-01 | |
| 3 | S3A - S3B Toxicokinetics and Pharmacokinetics/毒代动力学和药代动力学 | ||||||
| S3A: Note for Guidance on Toxicokinetics: The Assessment of Systemic Exposure in Toxicity Studies | S3A:毒代动力学指导原则说明:毒性研究中的全身暴露量评价 | 阶段5 | 1994.10.27 | 有 | 2019-11-05 | 2020-05-01 | |
| S3A Implementation Working Group Questions and Answers | S3A 问答 毒代毒代动力学指导原则说明:毒性研究中的全身暴露量评价-聚焦于微量采样 | 阶段3 | 2016.1.19 | 有 | 2019-11-05 | 2020-05-01 | |
| S3B: Pharmacokinetics Guidance for Repeated Dose Tissue Distribution Studies | S3B:药代动力学:重复给药的组织分布研究指导原则 | 阶段5 | 1994.10.27 | 有 | 2019-11-05 | 2020-05-01 | |
| 4 | S4 Toxicity Testing/毒性试验 | ||||||
| S4: Duration of Chronic Toxicity Testing in Animals (Rodent and Non Rodent Toxicity Testing) | S4:动物慢性毒性试验的期限(啮齿类和非啮齿类) | 阶段5 | 1998.9.2 | 有 | 2019-11-05 | 2020-05-01 | |
| 5 | S5 Reproductive Toxicology/生殖毒性 | ||||||
| S5(R2):Detection of Toxicity to Reproduction for Medicinal Products & Toxicity to Male Fertility | S5(R2): 检测药品的生殖毒性以及对雄性生殖能力的毒性 | 阶段5 | 2000.11 | 有 | |||
| S5(R3): Detection of Reproductive and Developmental Toxicity for Human Pharmaceuticals | S5(R3):人用药物生殖与发育毒性检测 | 阶段5 | 2020.2.18 | 有 | 2021-01-21 | 2021-01-21 | |
| 6 | S6 Biotechnological Products/生物技术产品 | ||||||
| S6(R1): Preclinical Safety Evaluation of Biotechnology-Derived Pharmaceuticals | S6(R1):生物制品的临床前安全性评价 | 阶段5 | 2011.6.12 | 有 | 2019-11-05 | 2020-05-01 | |
| 7 | S7A - S7B Pharmacology Studies/药理学研究 | ||||||
| S7A: SAFETY PHARMACOLOGY STUDIES FOR HUMAN PHARMACEUTICALS | S7A:人用药品安全药理学试验指导原则 | 阶段5 | 2000.11.8 | 有 | 2019-11-05 | 2020-05-01 | |
| S7B: The Non-Clinical Evaluation of the Potential for Delayed Ventricular Repolarization (QT Interval Prolongation) by Human Pharmaceuticals | S7B:人用药品延迟心室复极化(QT间期延长)潜在作用的非临床评价指导原则 | 阶段5 | 2005.5.12 | 有 | 2019-11-05 | 2020-05-01 | |
| 8 | S8 Immunotoxicology Studies 免疫毒理学研究 | ||||||
| S8: Immunotoxicity Studies for Human Pharmaceuticals | S8:人用药物免疫毒性研究 | 阶段5 | 2005.9.15 | 有 | 2019-11-05 | 2020-05-01 | |
| 9 | S9 Nonclinical Evaluation for Anticancer Pharmaceuticals/抗癌药物的非临床评价 | ||||||
| S9: Nonclinical Evaluation for Anticancer Pharmaceuticals | S9:抗肿瘤药物非临床评价指导原则 | 阶段5 | 2009.10.29 | 有 | 2019-11-05 | 2020-05-01 | |
| S9 Implementation Working Group Questions and Answers | S9:抗肿瘤药物非临床评价指导原则问答 | 阶段3 | 2016.6.8 | 有 | 2019-11-05 | 2020-05-01 | |
| 10 | S10 Photosafety Evaluation/光安全性评价 | ||||||
| S10: Photosafety Evaluation of Pharmaceuticals | S10:药物光安全评价 | 阶段5 | 2013.11.13 | 有 | 2019-11-05 | 2020-05-01 | |
| 11 | S11 Nonclinical Safety Testing In Support of Development of Paediatric Pharmaceuticals/儿科用药 | ||||||
| S11:Nonclinical Safety Testing In Support of Development of Paediatric Pharmaceuticals | S11:支持儿科用药开发的非临床安全性评价 | 阶段5 | 2020.4.14 | 有 | 2021-01-21 | 2021-01-21 | |
| 12 | S12:Nonclinical Biodistribution Considerations For Gene Therapy Products/基因治疗产品非临床生物分布的考虑 | ||||||
| S12:Nonclinical Biodistribution Considerations For Gene Therapy Products | S12:基因治疗产品非临床生物分布的考虑 | 阶段5 | 2023.3.14 | 有 | 2023-09-04 | 2023-09-04 | |
| 有效性(Efficacy Guidelines) | |||||||
| 1 | E1 Clinical Safety for Drugs used in Long-Term Treatment/长期使用的药物的临床安全性 | ||||||
| E1: The extent of Population Exposure to Assess Clinical Safety for Drugs Intended for Long-term Treatment of Non-life-threatening Conditions | E1:人群暴露程度:评估非危及生命性疾病长期治疗药物的临床安全性 | 阶段5 | 1994.10.27 | 有 | 2019-11-05 | 2020-05-05 | |
| 2 | E2A - E2F Pharmacovigilance/药物警戒性 | ||||||
| E2A: Clinical Safety Data Management: Definitions and Standards for Expedited Reporting | E2A: 临床安全性数据管理:快速报告的定义和标准 | 阶段5 | 1994.10.27 | 有 | |||
| E2B(R3):Implementation Guide for Electronic Transmission of Individual Case Safety Reports (ICSRs) E2B(R3) Data Elements and Message Specification | E2B(R3):个例安全报告(ICSR)电子传输执行指导原则 E2B(R3)数据元素和信息规范元素 (中文版:征求意见稿) | 阶段5 | 2016.11.10 | 有 | |||
| E2B(R3) QA document_v2_1 | E2B(R3) 问答文件(中文版:征求意见稿) | 阶段5 | 2017.6.1 | 有 | |||
| E2C(R2): Periodic Benefit-Risk Evaluation Report | E2C(R2): 定期获益—风险评估报告 | 阶段5 | 2012.12.17 | 有 | 2020-07-17 | 2020-07-17 | |
| E2C(R2) Implementation Working Group Questions & Answers | E2C(R2)实施工作组 问答部分 | 阶段5 | 2014.3.31 | 有 | 2020-07-17 | 2020-07-17 | |
| E2D: Post-Approval Safety Data Management: Definitions and Standards for Expedited Reporting | E2D: 上市后安全性数据的管理:快速报告的定义和标准(中文版:征求意见稿) | 阶段5 | 2003.11.12 | 有 | |||
| E2E: Pharmacovigilance Planning | E2E:药物警戒计划 | 阶段5 | 2004.11.18 | 有 | 2019-11-05 | 2020-02-05 | |
| E2F:Example DSUR – Phase III Investigational Drug | E2F:研发期间安全性更新报告示例 | 2010.10.05 | 有 | 2019-11-05 | 2019-11-05 | ||
| E2F: Development Safety Update Report | E2F:研发期间安全性更新报告 | 阶段5 | 2010.8.17 | 有 | |||
| 3 | E3 Clinical Study Reports/临床研究报告 | ||||||
| E3: Structure and Content of Clinical Study Reports | E3:临床研究报告的结构与内容 | 阶段5 | 1995.11.30 | 有 | 2019-11-05 | 2020-05-05 | |
| E3 Questions & Answers (R1) : Structure and Content of Clinical Study Reports | E3:临床研究报告的结构和内容问与答(R1) | 阶段5 | 2012.7.6 | 有 | 2019-11-05 | 2020-05-05 | |
| 4 | E4 Dose-Response Studies/剂量反应研究 | ||||||
| E4: Dose-Response Information to Support Drug Registration | E4:药品注册所需的量效关系信息 | 阶段5 | 1994.3.10 | 有 | 2019-11-05 | 2020-05-05 | |
| 5 | E5 Ethnic Factors/种族因素 | ||||||
| E5(R1): Ethnic Factors in the Acceptability of Foreign Clinical Data | E5(R1):接受国外临床试验数据的种族因素 | 阶段5 | 1998.2.5 | 有 | 2019-11-05 | 2019-11-05 | |
| E5 Implementation Working Group Questions & Answers (R1) | E5:接受国外临床试验数据的种族因素问答(R1) | 阶段5 | 2006.6.2 | 有 | |||
| 6 | E6 GCP/药物临床试验管理规范 | ||||||
| E6(R1): Guideline for Good Clinical Practice | E6(R1):药物临床试验管理规范指导原则 | 阶段5 | 1996.6.10 | 有 | |||
| E6(R2):Integrated Addendum to Good Clinical Practice (GCP) | E6(R2):药物临床试验管理规范综合附录 | 阶段5 | 2016.11.9 | ||||
| 7 | E7 Clinical Trials in Geriatric Population/老人中开展的临床试验 | ||||||
| E7: Studies in Support of Special Populations: Geriatrics | E7:特殊人群的研究:老年医学 | 阶段5 | 1993.6.24 | 有 | 2019-11-05 | 2020-05-05 | |
| E7 Questions & Answers | E7 特殊人群的研究:老年医学问答 | 阶段5 | 2010.7.6 | 有 | 2019-11-05 | 2020-05-05 | |
| 8 | E8 General Considerations for Clinical Trials/临床试验的一般性考虑 | ||||||
| E8(R1): General Considerations for Clinical Trials | E8(R1):临床试验的一般考虑 | 阶段5 | 2021.10.06 | 有 | 2022-08-01 | 2023-07-31 | |
| 9 | E9 Statistical Principles for Clinical Trials/临床试验的统计原则 | ||||||
| E9: Statistical Principles for Clinical Trials | E9:临床试验的统计学原则 | 阶段5 | 1998.2.5 | 有 | 2019-11-05 | 2020-05-05 | |
| E9(R1): Addendum on E stimands and Sensitivity Analysis in Clinical Trials | E9(R1):临床试验中的估计目标与敏感性分析(E9指导原则增补文件) | 阶段5 | 2019.11.20 | 有 | 2021-01-21 | 2022-01-21 | |
| 10 | E10 Choice of Control Group in Clinical Trials/试验中对照组的选择 | ||||||
| E10: Choice of Control Group and Related Issues in Clinical Trials | E10:临床试验中对照组的选择和相关问题 | 阶段5 | 2000.7.20 | 有 | 2019-11-05 | 2020-05-05 | |
| 11 | E11 Clinical Trials in Pediatric Population/儿童人群临床研究 | ||||||
| E11(R1): Addendum: Clinical Investigation of Medicinal Products in the Pediatric Population | E11(R1):用于儿科人群的医学产品的药物临床研究 | 阶段5 | 2017.8.18 | 有 | 2019-11-05 | 2020-05-05 | |
| 12 | E12 Clinical Evaluation by Therapeutic Category/根据治疗类别进行临床评价 | ||||||
| E12A: Principles for Clinical Evaluation of New Antihypertensive Drugs | E12A:抗高血压新药临床评价原则 | 阶段5 | 2000.3.2 | 有 | 2019-11-05 | 2020-05-05 | |
| 13 | E14 Clinical Evaluation of QT/QT临床评价 | ||||||
| E14: The Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs | E14:非抗心律失常药物QT/QTc间期延长及致心律失常潜力的临床评价 | 阶段5 | 2005.5.12 | 有 | 2022-08-01 | 2023-07-31 | |
| E14 Implementation Working Group Questions & Answers (R3) | E14 实施工作组 问答部分(R3) | 阶段5 | 2015.12.10 | 有 | |||
| E14/S7B: Clinical and Nonclinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential Questions and Answers | E14/S7B: QT/QTc 间期延长及潜在致心律失常作用的临床和非临床评价问答 | 阶段5 | 2022.02.21 | 有 | 2023-03-20 | 2023-07-31 | |
| 14 | E15 Definitions in Pharmacogenetics/Pharmacogenomics/药物基因组学以及遗传药理学相关定义 | ||||||
| E15: Definitions for Genomic Biomarkers, Pharmacogenomics, Pharmacogenetics, Genomic Data and Sample Coding Categories | E15:基因组生物标志物、药物基因组学、遗传药理学、基因组数据和样本编码分类的定义 | 阶段5 | 2007.11.1 | 有 | 2019-11-05 | 2020-05-05 | |
| 15 | E16 Qualification of Genomic Biomarkers/基因组生物标志物的合格条件 | ||||||
| E16: Biomarkers Related to Drug or Biotechnology Product Development: Context, Structure and Format of Qualification Submissions | E16:药物或生物技术产品开发相关的生物标志物:资格认定申请的背景资料、结构和格式 | 阶段5 | 2010.8.20 | 有 | 2019-11-05 | 2020-05-05 | |
| 16 | E17 Multi-Regional Clinical Trials/多地区临床试验 | ||||||
| E17: General principle on planning and Designing Multi-Regional Clinical Trials | E17:多区域临床试验计划与设计的一般原则 | 阶段5 | 2019.11.12 | 有 | 2019-11-05 | 2019-11-05 | |
| 17 | E18 Genomic Sampling/基因组取样 | ||||||
| E18: Genomic Sampling and Management of Genomic Data | E18:基因组采样和基因组数据管理指导原则(中文翻译公开征求意见稿) | 阶段5 | 2015.12.10 | 有 | 2021-11-01 | 2022-05-01 | |
| 18 | E19:A Selective Approach To Safety Data Collection In Specific Late-Stage Pre-approval Or Post-Approval Clinical Trials/在特定的上市前后期或上市后临床试验中选择性收集安全性数据 | ||||||
| E19:A Selective Approach To Safety Data Collection In Specific Late-Stage Pre-approval Or Post-Approval Clinical Trials | E19:在特定的上市前后期或上市后临床试验中选择性收集安全性数据 | 阶段5 | 2022.09.27 | 有 | 2023-04-21 | 2023-10-21 | |
| 多学科(Multidisciplinary Guidelines) | |||||||
| 1 | M1 MedDRA Terminology 监管活动医学词典 | ||||||
| MedDRA Points to Consider Companion Document | MedDRA ® 数据检索和展示: 考虑要点 | 2018.06 | 有 | ||||
| MedDRA Term Selection: Points to Consider | MedDRA ® 术语选择: 考虑要点 | 2018.09.01 | 有 | ||||
| MedDRA Best Practices | MedDRA ® 最佳规范 | 2018 | 有 | ||||
| 2 | M2 Electronic Standards 电子标准 | ||||||
| M2:Electronic Standards for the Transfer of Regulatory Information Final Concept Paper | M2:监管信息电子传输标准 最终概念文件 | 1994.10.27 | 有 | ||||
| Electronic Standards for the Transfer of Regulatory Information (ESTRI) General Recommendation - Procedure | 监管信息电子传输标准 一般性建议-程序 | 2015.6.11 | 有 | ||||
| Electronic Standards for the Transfer of Regulatory Information (ESTRI)-Gateway | 监管信息电子传输标准 一般性建议-ESTRI网关 | 2015.6.11 | 有 | ||||
| Electronic Standards for the Transfer of Regulatory Information (ESTRI) File Format Recommendation – PDF | 监管信息电子传输标准 文件格式建议-PDF | 2011.4.5 | 有 | ||||
| Electronic Standards for the Transfer of Regulatory Information (ESTRI) File Format Recommendation – XML | 监管信息电子传输标准 文件格式建议-XML | 2005.11.10 | 有 | ||||
| Electronic Standards for the Transfer of Regulatory Information (ESTRI) File Format Recommendation – PDF/A | 监管信息电子传输标准 文件格式建议-PDF/A | 2014.6.2 | 有 | ||||
| Electronic Standards for the Transfer of Regulatory Information (ESTRI)File Format Recommendation – DOCX | 监管信息电子传输标准 文件格式建议-DOCX | 2015.6.11 | 有 | ||||
| Electronic Standards for the Transfer of Regulatory Information Controlled Vocabularies Recommendation - Genericode | 监管信息电子传输标准 受控词汇建议-通用编码 | 2015.6.11 | 有 | ||||
| Electronic Standards for the Transfer of Regulatory Information Information Transfer Recommendation – EDIINT V3.0 | 监管信息电子传输标准 信息传输建议-EDIINT V3.0 | 2018.6.7 | 有 | ||||
| Electronic Standards for the Transfer of Regulatory Information (ESTRI) File Integrity – MD5 | 监管信息电子传输标准 文件完整性-MD5 | 2010.6.10 | 有 | ||||
| Electronic Standards for the Transfer of Regulatory Informaation (ESTRI) File Integrity Recommendation - SHA-256 | 监管信息电子传输标准 文件完整性建议-SHA-256 | 2015.6.11 | 有 | ||||
| M2:Glossary of Terms and Abbreviations | M2:术语和缩略语词汇表 | 2015.6.11 | 有 | ||||
| M2:File Format Criteria | M2:文件格式标准 | 2014.11.10 | 有 | ||||
| Use of OIDs & UUIDs in ICH Messages | OID和UUID在ICH消息中的应用 | 2015.6.11 | 有 | ||||
| 3 | M3 Nonclinical Safety Studies 非临床研究 | ||||||
| M3(R2) Questions and Answers (R2) | M3(R2)问答 (R2) | 阶段5 | 2012.3.5 | 有 | 2021-11-01 | 2021-11-01 | |
| M3(R2): Guidance on Nonclinical Safety Studies for the Conduct of Human Clinical Trials and Marketing Authorization for Pharmaceuticals | M3(R2):支持药物进行临床试验和上市的非临床安全性研究指导原则 | 阶段5 | 2009.6.11 | 有 | 2021-11-01 | 2021-11-01 | |
| 4 | M4 : The Common Technical Document 通用技术文件 | ||||||
| M4 (R4): Organization of the Common Technical Document for the Registration of Pharmaceuticals for Human Use | M4(R4):人用药物注册通用技术文档的组织(中文版:征求意见稿) | 阶段5 | 2016.6.15 | 有 | |||
| M4 Implementation Working Group Questions & Answers (R3) | M4执行工作组问答(R3)(中文版:征求意见稿) | 阶段5 | 2004.6.10 | 有 | |||
| The Common Technical Document for the Registration of Pharmaceuticals for Human Use: Quality – M4Q(R1) | M4Q(R1):人用药物注册通用技术文档:药学部分(中文版:征求意见稿) | 阶段5 | 2002.9.12 | 有 | |||
| M4Q Implementation Working Group Questions & Answers (R1) | M4Q执行工作组问答(R1)(中文版:征求意见稿) | 阶段5 | 2003.7.17 | 有 | |||
| The Common Technical Document for the Registration of Pharmaceuticals for Human Use: Safety – M4S(R2) | M4S(R2):人用药物注册通用技术文档:安全性部分(中文版:征求意见稿) | 阶段5 | 2002.12.20 | 有 | |||
| M4S Implementation Working Group Questions & Answers (R4) | M4S执行工作组问答 (R4)(中文版:征求意见稿) | 阶段5 | 2003.11.11 | 有 | |||
| Efficacy- M4E(R2) | M4E(R2):人用药物注册通用技术文档:有效性部分(中文版:征求意见稿) | 阶段5 | 2016.6.15 | 有 | |||
| M4E Implementation Working Group Questions & Answers (R4) | M4E执行工作组问答(R4)(中文版:征求意见稿) | 阶段5 | 2004.6.10 | 有 | |||
| 5 | M5 Data Elements and Standards for Drug Dictionaries 药物词典的数据要素和标准 | ||||||
| The Re-development of the Standard for E2B(R3) and the Development of Standards for the Identification of Medicinal Products (IDMP)(ICH M5) | ICH M5: E2B(R3)标准的再制定及医药产品鉴定标准的制定 | 2010.11.1 | |||||
| ICH E2B(R3) Implementation Working Group ICH E2B(R3) Guideline: Electronic Transmission of Individual Case Safety Reports (ICSRs) | E2B(R3)实施工作组 个例病例安全报告的电子传输 问答部分 | 2.0版本 | 2016.11.10 | ||||
| Appendix I (B) to the Implementation Guide for Electronic Transmission of Individual Case Safety Reports (ICSRs) | 个例病例安全报告的电子传输实施指南附录 I (B) | 2.02版本 | 2016.11.10 | ||||
| Appendix I (G) to the Implementation Guide for Electronic Transmission of Individual Case Safety Reports (ICSRs) | 个例病例安全报告的电子传输实施指南附录 I (G) | 1.02版本 | 2016.11.10 | ||||
| Implementation Guide for Electronic Transmission of Individual Case Safety Reports (ICSRs) | 个例病例安全报告的电子传输实施指南 | 5.02版本 | 2016.11.10 | ||||
| 6 | M6 Gene Therapy 基因治疗 | ||||||
| Final Concept Paper M6: Guideline on Virus and Gene Therapy Vector Shedding and Transmission | M6: 病毒和基因治疗载体的脱落和传播 终版概念文件 | 2009.8.26 | |||||
| General Principles to Address Virus and Vector Shedding | 解决病毒和基因治疗载体脱落的基本原则 | 2009.6 | |||||
| An inventory of shedding data from clinical gene therapy trials | 临床基因疗法试验脱落数据目录 | 2007.7.30 | |||||
| Final Business Plan M6: Guideline on Virus and Gene Therapy Vector Shedding and Transmission | M6: 病毒和基因治疗载体的脱落和传播 终版业务计划 | 2009.8.27 | |||||
| 7 | M7 Genotoxic Impurities 遗传毒性杂质 | ||||||
| M7: Assessment and Control of DNA Reactive (Mutagenic) Impurities in Pharmaceuticals to Limit Potential Carcinogenic Risk | M7:评估和控制药物中的DNA活性(致突变)杂质以限制潜在的致癌风险 | 阶段5 | 2014.6.23 | 有 | |||
| M7(R1): Addendum to M7: Assessment and Control of DNA Reactive (Mutagenic) Impurities in Pharmaceuticals to Limit Potential Carcinogenic Risk | M7(R1):评估和控制药物中DNA反应性(致突变)杂质以限制潜在致癌风险 | 阶段5 | 2017.3.31 | 有 | |||
| M7(R2):Assessment and Control of DNA Reactive (Mutagenic) Impurities in Pharmaceuticals to Limit Potential Carcinogenic Risk | M7(R2):评估和控制药物中DNA反应性(致突变)杂质以限制潜在致癌风险 | 阶段5 | 2023.4.3 | 有 | 2024-01-03 | 2024-07-03 | |
| M7(R2):Questions and Answers | M7(R2):问答文件 | 阶段5 | 2022.5.24 | 有 | |||
| Addendum to M7(R2) :Application of The Principles of the ICH M7 Guideline to Calculation of Compound-Specific Acceptable Intakes | M7(R2)附录:ICH M7原则在化合物可接受摄入量计算中的应用 | 阶段5 | 2023.4.3 | 有 | |||
| 8 | M8 Electronic Common Technical Document (eCTD) 电子通用技术文件 | ||||||
| Electronic Common Technical Document Specification V3.2.2 | 电子通用技术文件规范 V3.2.2 | 2008.7.16 | |||||
| M8 : Electronic Common Technical Document Concept Paper | M8: 电子通用技术文件 概念文件 | 2015.12.9 | |||||
| ICH M8 EWG/IWG Work Plan | M8: 电子通用技术文件 工作计划 | 2017.3.13 | |||||
| Support Documentation for M8: eCTD EWG eCTD v4.0 Implementation Package v1.2 | M8:eCTD专家工作组eCTD v4.0实施包 v1.2 支持性证明文件 | 2016.11 | |||||
| Orientation Material forM8: eCTD EWG eCTD v4.0 Implementation Package v1.2 | M8:eCTD专家工作组eCTD v4.0实施包 v1.2 培训材料 | 2016.11 | |||||
| ICH Electronic Common Technical Document (eCTD) v4.0 Implementation Guide v1.2 | ICH eCTD v4.0 实施指南 v1.2 | 2016.11.10 | |||||
| eCTD v4.0 Implementation Package v1.2 | eCTD v4.0 实施包 v1.2 | ||||||
| USFDA eCTD v4.0 Implementation Package History v1.1 | 美国FDA eCTD v4.0 实施包历史 v1.1 | ||||||
| USFDA Module 1 Electronic Common Technical Document (eCTD) v4.0 Implementation Guide v1.1 | 美国FDA 模块1 eCTD v4.0 实施指南 v1.1 | 2017.2.20 | |||||
| ICH eCTD v4.0 Requirements | ICH eCTD v4.0 要求 | ||||||
| ICH M8 Expert Working Group Specification for Submission Formats for eCTD | eCTD提交格式规范 | 2016.11.10 | |||||
| Change Control Process for the eCTD | eCTD变更控制过程 | 2017.4 | |||||
| Request for change | 请求变更表 | ||||||
| 9 | M9 Biopharmaceutics Classification System-based Biowaivers 基于生物药剂学分类系统的生物豁免 | ||||||
| M9: Biopharmaceutics Classification System-based Biowaivers | M9:基于生物药剂学分类系统的生物等效性豁免 | 阶段5 | 2019.11.20 | 有 | 2021-04-28 | 2021-10-28 | |
| M9 Questions and Answers | M9问答文件 | 阶段5 | 2019.11.20 | 有 | 2021-04-28 | 2021-10-28 | |
| 10 | M10 Bioanalytical Method Validation and Study Sample Analysis 生物分析方法验证及样品分析 | ||||||
| M10 Bioanalytical Method Validation and Study Sample Analysis | M10:生物分析方法验证及样品分析 | 阶段5 | 2022.05.24 | 有 | 2023-06-29 | 2023-07-29 | |
| M10 Questions and Answers(Q&As) | M10问答文件 | 阶段5 | 2022.11.16 | 有 | 2023-06-29 | 2023-07-29 | |
| M10 Frequently Asked Questions (FAQs) | M10常见问题解答文件 | 阶段5 | 2022.11.11 | 有 | 2023-06-29 | 2023-07-29 | |
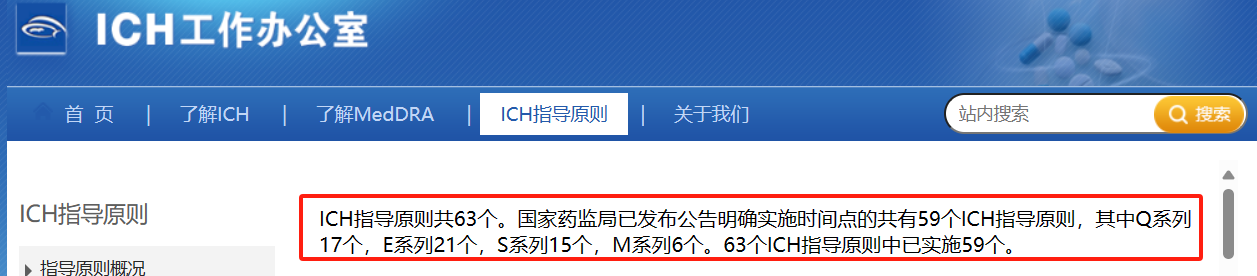
ICH官网显示指导原则共63个,Q系列14个,S系列12个,E系列22个,M系列15个。网址如下:https://www.ich.org/page/ich-guidelines
评论
匿名
提交
取消
匿名
{{item_parent.created_at}}
置顶
批准
驳回
编辑
等待审核
已驳回
回复
{{item_parent.show_reply_list ? '收起回复' : '查看回复'}}({{item_parent.children.length}})
编辑
提交
取消
写回复
匿名
提交
取消
{{item_children.from_user}} 回复 {{item_children.to_user}}
{{item_children.created_at}}
批准
驳回
编辑
等待审核
已驳回
回复
编辑
提交
取消
写回复
匿名
提交
取消
这{{threadTextType}}正{{isAdminText}}
举报
提交
取消
为帮助审核人员更快处理,请填写举报原因:
举报
提交
取消
为帮助审核人员更快处理,请填写举报原因: