上个月EDQM出了一个与此相关的问答:How to present the specification for a substance in a CEP application?
The specification for the substance should primarily demonstrate compliance with the Ph. Eur. monograph and with European regional requirements. Therefore, it should not include quality attributes that are included only for the purpose of demonstrating compliance with pharmacopoeias other than the Ph. Eur. Including such quality attributes may:
- Cause a delay in the CEP being granted or revisions accepted.
- Increase the likelihood of more frequent revisions of the CEP during its lifecycle.
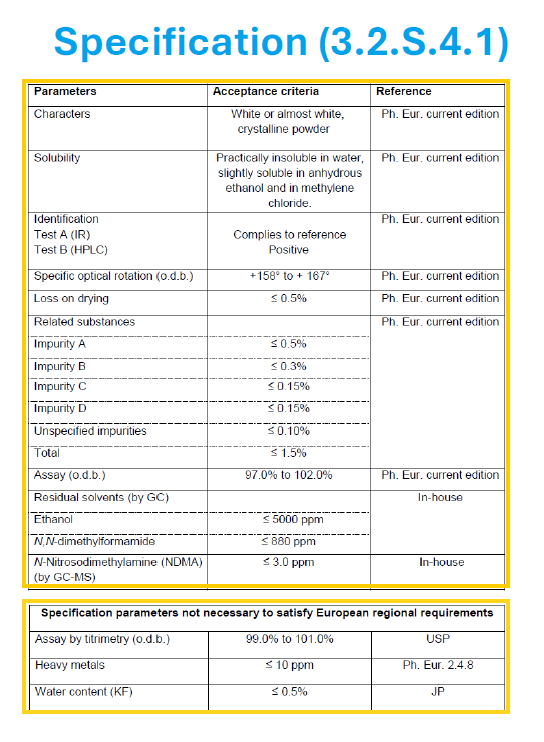
Despite this, if a CEP applicant/holder decides to include quality attributes that are intended to satisfy a regulatory requirement in another region (i.e. non-European regional requirement) in their specification, they should present them separately (e.g. in an additional table) and identify them accordingly as shown below:
Specification parameters not necessary to satisfy European regional requirements | ||
Assay (by titration) | 99.0% to 101.0% | USP |
Heavy metals | ≤ 10 ppm | Ph. Eur. 2.4.8 |
Water content (by KF) | ≤ 0.5% | JP |
This approach helps maintain clarity and avoids unnecessary burden during evaluation of the CEP application and eases lifecycle management and the use of the CEP.
这{{threadTextType}}正{{isAdminText}}
为帮助审核人员更快处理,请填写举报原因:
为帮助审核人员更快处理,请填写举报原因: